
A series of investigations conducted by Kaiser Health News (KHN) discovered that for 20 years a hidden database used by the Food and Drug Administration allowed makers of implant devices and medical tools to secretly log more than 1.1 million malfunctions and injuries just since 2016.
ARS Technica reported about KHN’s discovery of over 50,000 hidden reports on heart devices.
The device—the Sprint Fidelis, made by Medtronic—consists of a pair of wires and a defibrillator to jolt the heart into a regular rhythm. But doctors found that it was giving patients random, harmful zaps and sometimes failed during actual cardiac emergencies.
Medtronic recalled the device in 2007 but only after it was implanted in around 268,000 patients. Many of those patients have since faced the ghastly choice of learning to live with the faulty device or undergoing an invasive, risky—sometimes deadly—surgery to remove it. According to the KHN investigation, they’ve been making that choice without information from the 50,000 incident reports.
ARS
The FDA has a “public-facing” database called MAUDE, Manufacturer and User Facility Device Experience, where manufacturers like Sprint Fidelis would report malfunctions.
“However, the agency quietly set up an “alternative summary reporting” repository and for decades granted reporting exemptions for a wide variety of medical devices. The agency has since piled up millions of incident reports out of public view. That includes the 50,000 reports for the Sprint Fidelis. Since 2016, the FDA’s repository accrued at least 1.1 million reports of incidents and injuries overall from malfunctioning medical devices, KHN found.”
The alternative reporting repository was ostensibly set up to cut down on paperwork for redundant incident reports. But its existence and use has been kept in the shadows, with many doctors, consumer advocates, and even some high-ranking employees at the FDA kept completely unaware of its existence. Now that they are aware, doctors and advocates say the concealed reports have kept necessary safety data from patients trying to accurately gauge and weigh their risks.
…
The list of exempted devices includes common and controversial devices. Some of these are typically implanted into patients and used in common surgeries, such as pelvic mesh, surgical staples, balloon pumps for improving circulation, breathing machines, and breast implants.
KHN first reported the findings of their investigations on March 7th:
Hidden FDA Reports Detail Harm Caused By Scores Of Medical Devices –
The Food and Drug Administration has let medical device companies file reports of injuries and malfunctions outside a widely scrutinized public database, which leave doctors and medical sleuths in the dark.
Dr. Douglas Kwazneski was helping a Pittsburgh surgeon remove an appendix when something jarring happened. The surgical stapler meant to cut and seal the tissue around the appendix locked up.
Kwazneski later turned to the Food and Drug Administration’s public database that tracks medical device failures and “there was nothing,” he said. Yet when he surveyed leading surgeons on the matter, he discovered that more than two-thirds had experienced a stapler malfunction, or knew a peer who did. Such failures can have deadly consequences.
Kwazneski had no idea the FDA had quietly granted the makers of surgical staplers a special “exemption” allowing them to file reports of malfunctions in a database hidden from doctors and from public view.
“I don’t want to sound overdramatic here, but it seemed like a cover-up,” said Kwazneski, who practiced in Pasco County, Fla., from 2016 through earlier this year.
The FDA has built and expanded a vast and hidden repository of reports on device-related injuries and malfunctions, a Kaiser Health News investigation shows. Since 2016, at least 1.1 million incidents have flowed into the internal “alternative summary reporting” repository, instead of being described individually in the widely scrutinized public database known as MAUDE, which medical experts trust to identify problems that could put patients in jeopardy.
Deaths must still be reported in MAUDE. But the hidden database has included serious injury and malfunction reports for about 100 medical devices, according to the FDA, many implanted in patients or used in countless surgeries. They have included surgical staplers, balloon pumps snaked into vessels to improve circulation and mechanical breathing machines.
Kaiser Health News
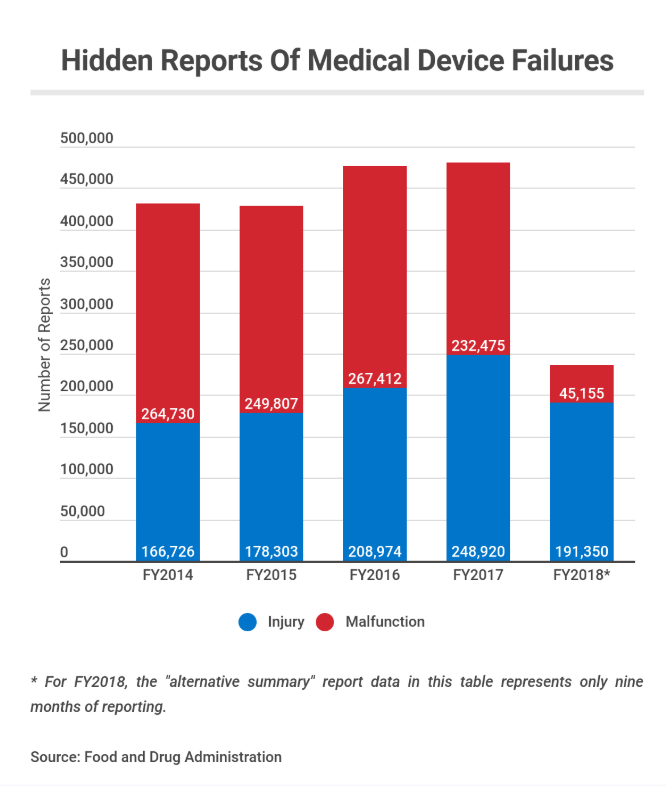
Amid the growing scandal, KHN recently reported, the FDA “announced it is shutting down its controversial “alternative summary reporting” program and ending its decades-long practice of allowing medical device makers to conceal millions of reports of harm and malfunctions from the general public.”
For a deep dive read on this worldwide issue, see the International Consortium of Investigative Journalists (ICIJ) – Implant Files.
