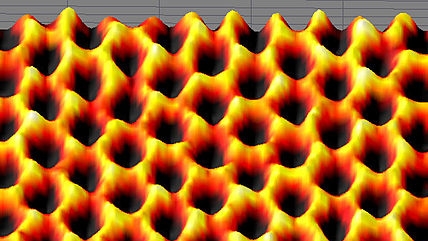
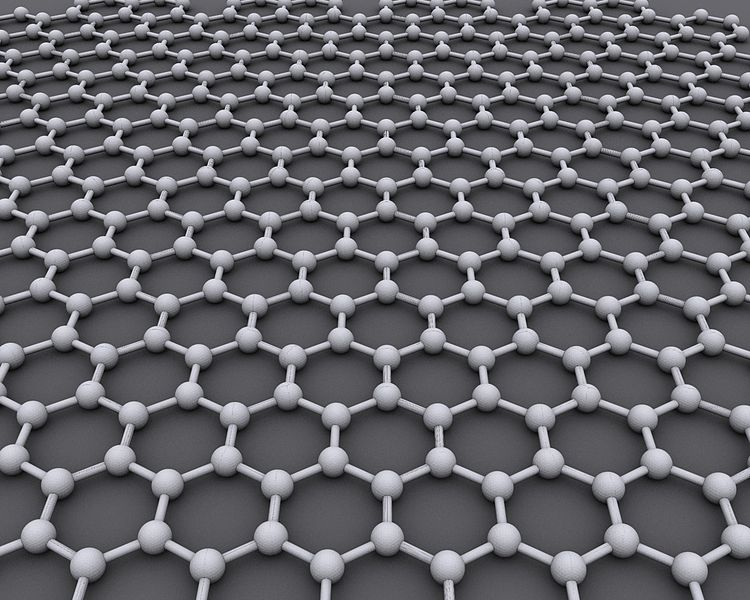
Diamonds, pencil lead, and a material called graphene are three allotropes of carbon. That is, they are different tangible forms of the same chemical element: in this case, carbon. A diamond is a bunch of carbon atoms arranged in a pricey three-dimensional (3D) crystal. Pencil lead is an inexpensive 3D arrangement of carbon called graphite. Graphene is a relatively new “wonder material” of atoms arranged in a two dimensional (2D) crystal lattice. Think of it as a sheet of paper only one atom thick. Models of the ideal structure of graphene at the atomic level appear as a 2D hexagonal grid.
Graphene:
- Conducts electricity better than any other known substance (at room temperature)
- Can absorb and convert any wavelength of light into an electric current
- Is an excellent conductor of heat
- Is 200 times stronger than steel
- Is 1,000 times lighter than paper
- Is 98 percent transparent
- Is flexible enough to be used in clothing or printed on paper
- Is comprised of carbon, an element so abundant that it is the fourth most-common in the Universe.
Pure graphene is 100% carbon, but because each atom in the lattice has a free electron it can easily be alloyed to other materials, such as rubber.
Altough graphene had been identified previously, it wasn’t until 2004 that physicists Andre Geim and Konstantin Novoselov, working at the University of Manchester, UK, isolated graphene from a block of graphite using common transparent tape (what the British call “sticky tape”, and Americans call “scotch tape”). They also identified many of graphene’s wondrous properties. This unlikely achievement utilizing a sticky acetate earned them the 2010 Nobel Prize in Physics. Scientists and engineers have been working hard ever since, slowly transforming the science into commercial products. Many dicoveries have been made including a 2019 breakthrough at MIT where researchers ‘accidently’ discovered that graphene became a superconductor when two layers were laid one on top of the other at the “magic angle” of 1.1 degrees.
A few of the efforts to commercialize graphene include:
- Solid-state batteries
- Graphene transistors (replacing silicon)
- Computer chips (replacing silicon)
- Supercapacitors
- DNA sequencing
- Sensors/biosensors
- Clothing, wearable electronic devices
- Transparent touch screens (e.g., heads-up display)
- Solar cells/energy generation
- Hydrogen storage
- Water desalinization, filtering, and purification
- Sports equipment
- Much, much more.
It takes a great deal of time and effort to develop and bring new products to market from cutting edge science. For example, plastics were first developed in the 1920s, but several decades went by before plastic products were ubiquitous. Watch this well-done video, “Why graphene hasn’t taken over the world…yet” (7:43):
Question of the Night: You are offered a vault full of diamonds and other precious gems OR unlimited free access to the internet for life. You cannot ever sell the gems or make money off the internet access. Make your choice. Why?
