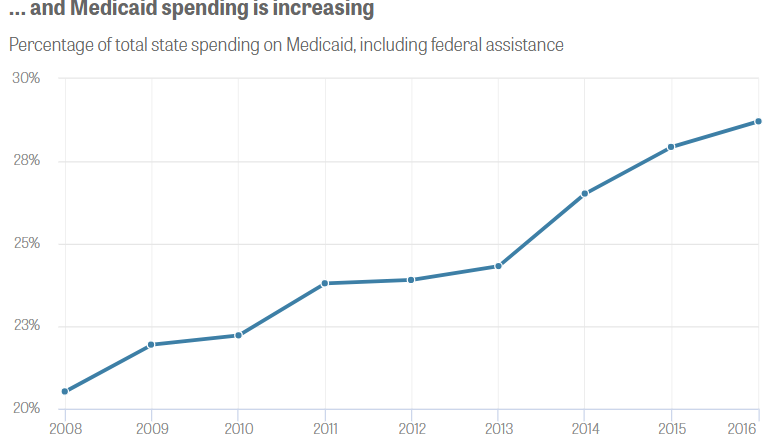
“Medicaid drug costs grew almost 50 percent per patient from 2008 through 2016, causing the program’s drug spending to nearly double to $31 billion. Such costs are helping drive up Medicaid expenses, which accounted for more than a quarter of 26 states’ budgets in 2016.”
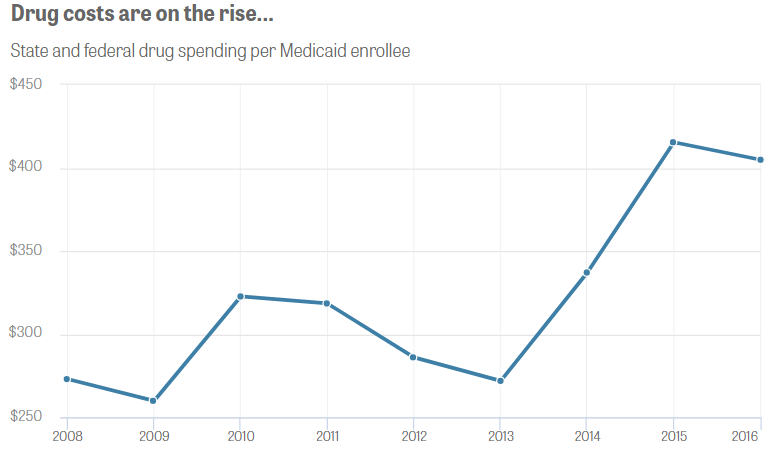
Source: Center for Public Integrity analysis of Medicaid state drug utilization data and Medicaid Budget and Expenditure System data. Graphic by Julia Donheiser.
This is just one conclusion of a recent joint investigative report by The Center for Public Integrity (CPI) and NPR they published in July of this year.
In October 2015 the DOJ announced the pharmaceutical maker Warner Chilcott pleaded guilty to a felony health care fraud scheme for illegal marketing and paying kickbacks to physicians for inducement to push their drugs and manipulating doctor’s authorization. They also unsealed indictment against its former president and three of its district managers who would face criminal charges.
This is where CPI & NPR pick up their story telling about a Warner Chilcott drug sales rep turned whistleblower.
“Eight months pregnant, the drug sales representative wore a wire for the FBI around her bulging belly as she recorded conversations with colleagues at a conference in Chicago. Her code name? Pampers.”
Lisa, who was involved in a whistleblower legal settlement, said her company “needs to be held accountable” and “if nobody else is going to do it” then she was.
Medicaid is the socialized medical insurance that uses state and federal tax payer dollars in a joint federal-state program to pay for over 76 million poor and or disabled Americans health care and drugs are a big part of that cost. CPI and NPR decided to investigate how states choose which drugs will be used, how that process is decided, and what influence drug manufactures play in that process.
And what they discovered is Warner Chilcott’s, now owned by Allergan, illegal and even criminal behaviors were just the tip of the iceberg.
States have a system, which includes what is called a Medicaid Drug Rebate program and the Drug Utilization Review program, they use for what drugs are approved for their Medicaid programs called the “preferred drug lists” for their outpatient prescription drugs program. This is typically in the form of a committee usually made up of volunteers from the medical community of physicians primarily from those who deal with Medicaid patients. They vote on which drugs will be included on this ‘preferred list’. If a drug doesn’t make the list then Medicaid patients, and drug manufacturers’ drug itself, are out of luck.
So drug makers around the country are, and have been for decades, dealing in influence peddling to get their drugs on the coveted government ‘preferred list’ and they’re paying doctors to speak to these drug committees, and in some cases paying the doctors who sit on the committees themselves who will vote on the drugs.
“…drug companies have infiltrated nearly every part of the process that determines how their drugs will be covered by taxpayers: giving free dinners and consulting gigs to many doctors on the obscure committees advising state Medicaid programs; asking speakers who don’t disclose their financial ties with drug companies to testify about their drugs; and paying for state Medicaid officials to attend all-inclusive conferences where they can mingle with drug representatives.”
One ‘official’ owned stock in several drug companies.
But the manufacturers said it’s the states’ fault because it was the drug’s industry’s ‘priority’ to make sure ‘patients have access to the medicines they need.”
From CPI:
Here’s how drug companies influence Medicaid decisions across the country:
Swarming the Medicaid drug committees | Enriching the decision-makers | Wining and dining state officials | Storming the legislatures| Skirting the preferred drug lists | The Broken System
______________________________________________________
To End on a (somewhat) Good Note
NPR reported Friday Texas is the latest state, along with Arizona, Colorado, and New York, to respond to CPI-NPR findings who are now requiring anyone who speaks before states’ Medicaid drug review boards “to disclose verbally and in writing if they have ‘directly or indirectly received payments or gifts’ from any pharmaceutical companies and to identify those firms.” They have also revised their policy that members who sit on their state review panel are to abstain from voting on a drug that would “present possible conflicts of interest, complete training on state transparency laws, and regularly sign an acknowledgment of the agency’s ethics policy.”
Opinion: I add (somewhat) because here were are, faced with one more ‘bigGovermment’ program ripe for corruption by un-elected, unaccountable bureaucrats responsible for what amounts to billions of tax payer dollars and here we are with them having to fix a problem they created in the first place.

1 Trackback / Pingback
Comments are closed.