
Every October 23 since 1991 has been a celebration of Mole Day throughout the world of chemistry. No, this isn’t about the subterranean burrowing mammal (a nerdy joke along these lines is required in every discussion of Avogadro’s Number). In chemistry, a mole is… well, more on that in a minute. Suffice to say for now, the mole is an essential tool of chemistry.
The science of chemistry is a cornerstone of our modern world, unappreciated by most consumers. We’re surrounded by products containing metals, plastics, coatings, rubber, synthetic fabrics, paper, electronics, and the list goes on and on. Even perfume and cologne blind us with chemical science. Hardly any of the objects we take for granted would be possible without chemistry. You probably can’t name a single thing you buy that does not depend on chemistry. Even if you bought organically-grown fruits and vegetables at your local farmer’s market, they were almost certainly brought there in a vehicle that uses petroleum products made possible by chemistry. Scientists and chemical engineers who make the products we use everyday labor in the background, sight unseen, and efforts mostly unrecognized by ordinary mortals. Mole Day is an unofficial holiday for them, but it’s also meant for chemistry students and their teachers and professors.
The idea of a Mole Day holiday was first floated in The Science Teacher journal, circa the early 1980s. A high school chemistry teacher in Prairie du Chien, Wisconsin, by the name of Maurice Oehler, thought it would be a fantastic way to promote the science of chemistry and get kids interested in the subject. Thus Mr. Oehler organized the National Mole Day Foundation (NMDF), established in 1991. Each year the NMDF embraces a different theme for Mole Day. This year, the theme is MOLEzilla! Why a theme is necessary or important, I don’t know, but there it is.
Mole Day isn’t a full 24 hours long; it’s actually only observed for 12 hours, from 6:02 am to 6:02 pm on October 23. That seems odd, doesn’t it? The purpose of this unusual arrangement is to shine a spotlight on Avogadro’s Number (also known as Avogadro’s constant or, ‘one mole’) the essential tool of chemistry mentioned earlier. Avogadro’s Number is written as 6.02×10^23, and read as 6.02 times 10, raised to the power of 23. That’s why the date (10/23) and time (6:02) were chosen.
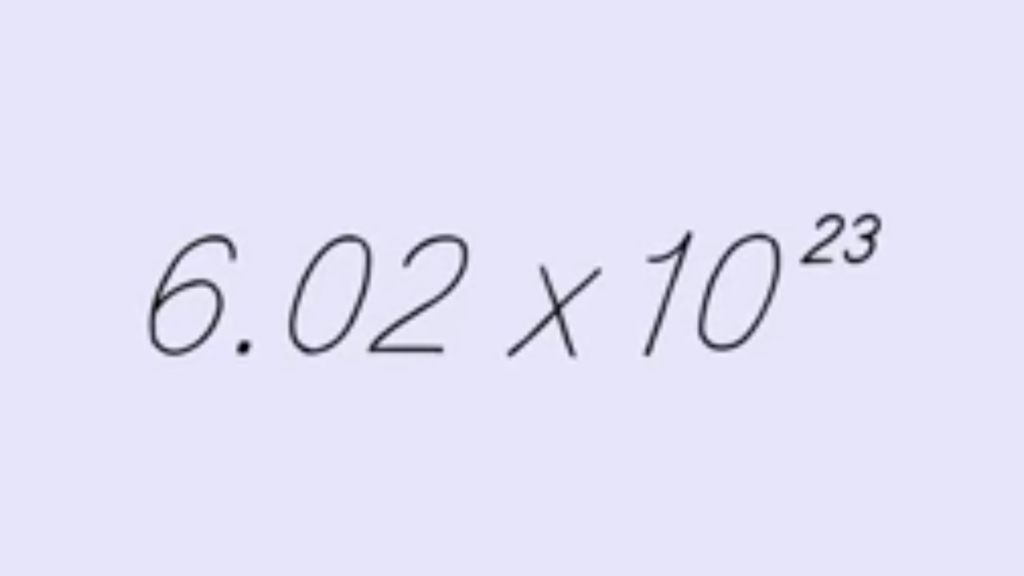
Actually, that’s the mathematically rounded version. A more precise figure for the mole (often abbreviated mol) is:
1 mol = 6.022,140,760×10^23
Which you may also see written as:
1 mol = 6.02214076E+23
How big a number is that?
602,214,076,000,000,000,000,000
Notice the 6 is followed by 23 decimal places, most of which are zeroes.
That’s over 602 sextillion (and no, I did not make that word up). Other ways to think about that number:
602 times a billion times a trillion.
602 billion × one trillion = 602 sextillion
Because, one billion (9 zeroes),
times one trillion (12 zeroes),
equals one sextillion (21 zeroes)
and 10x^21 = one sextillion.
(In case you’re wondering why I just wrote 10x^21 instead of 10x^23, it’s because the ’02’ in 602 are the 22nd and 23 decimal places).
Wow, 602 sextillion. That’s a big number. What does it apply to? Scientists and engineers use the mole as a measure of the number of atoms, ions, molecules, or electrons they’re dealing with. It’s used in a variety of ways, for example when mixing chemicals the ‘recipe’ might call for one mole of ‘A’ combined with two moles of ‘B’ to create a desired compound ‘C’. You may remember from chemistry class that:
2H2 + O2 → 2H2O
This chemical equation states that if you combine 2 moles of dihydrogen with one mole of dioxygen, the product of the chemical reaction will be 2 moles of water.
That’s an easy way of saying 12.04 sextillion molecules of the reactant dihydrogen combine with 6.02 sextillion molecules of the reactant dioxygen to form 12.04 sextillion molecules of water.
So, why is it called Avogadro’s Number? This excellent and entertaining video with plenty of nerdy references to a mammal called the mole may help shed further light on the subject. “How big is a mole? (Not the animal, the other one.) – Daniel Dulek” (4:33):
By the way, this also happens to be National Chemistry Week, sponsored by the American Chemical Society, and it always runs Sunday through Saturday of the week that includes October 23. Huzzah!
Question of the Night: Did you make a stink bomb or set anything on fire in chemistry class? If you never took chemistry, do you wish you had?

1 Trackback / Pingback
Comments are closed.